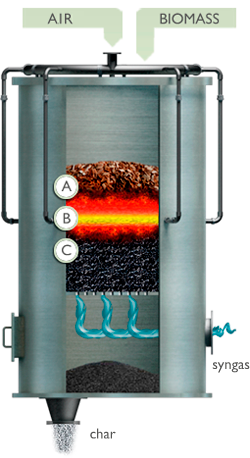
|
|
Gasification can be defined as the thermochemical conversion of biomass into a fuel gas. The industrial process involves two key sets of thermochemical reactions carried over at high temperature and with a controlled amount of oxygen: pyrolysis and gasification.
During pyrogasification, biomass long-chain chemical bonds are broken down into simpler molecules. As a result, the generic molecule of solid biomass is converted into three co-products:

| Gas |
| the gaseous fraction (syngas) composed of lighter, non-condensable molecules. It consists mainly of hydrogen, carbon monoxide, methane, carbon dioxide and nitrogen
|
| Liquid |
| the condensable vapors produced by the sudden depolymerization of cellulosic components (cellulose and hemicellulose). Once condensed, the mix of these hydrocarbon complexes with high heating value is generically referred to as tar or pyrolysis oil
|
| Solid |
| the part of unreacted charcoal (char), consists of carbon plus the ash initially contained in the biomass
|
The relative amounts of these three co-products depend on multiple factors, including the Heating Rate (speed at which the biomass is heated), the Equivalence Ratio (amount of oxygen used in the process), the process temperature and others. In our reactor these parameters are optimized for the production of syngas.
Thanks to the high efficiency of the process, over 90% of the energy content of the biomass is converted into pyrogasification co-products.
|